Abstract
We hypothesized that placental resistance was elevated and transfer reduced in cotyledons from intrauterine growth-restricted (IUGR) fetuses. We perfused 10 cotyledons from term, normally grown fetuses, six from preterm, normally grown fetuses with normal umbilical arterial end-diastolic velocities (EDV), and six from preterm IUGR fetuses (<3rd centile) with absent or reversed umbilical arterial EDV. Perfused cotyledons were pressure-fixed, and villi were observed by scanning electron microscopy. The groups did not differ in fetoplacental resistance at baseline; neither did they differ in the change in resistance that followed the administration of nitroglycerin or angiotensin II. The increase in resistance during hypoxia was similar in the two preterm groups but greater in the term than in the preterm normally grown group (p < 0.05). Groups did not differ in net maternofetal transfer of oxygen or glucose, or in clearance of aminoisobutyric acid or antipyrine. However, glucose consumption was doubled in cotyledons of preterm IUGR versus preterm normally grown fetuses (p < 0.05). Terminal villi of perfused cotyledons from preterm IUGR fetuses displayed less terminal villous branching and budding than preterm controls, as anticipated from previous work. IUGR fetuses with absent or reversed umbilical arterial EDV in vivo may have high placental resistance due to a vasoconstrictive rather than anatomic abnormality and an elevated placental glucose consumption that may impair glucose transfer.
Similar content being viewed by others
Main
Impaired growth in IUGR fetuses with absent or reversed EDV in the umbilical artery is probably secondary to inadequate exchange of nutrients and end products of metabolism at the placenta. Reduced exchange may be due in part to umbilical arterial blood flows that are low, often even when expressed on a per-kg fetal weight basis (1). Low flows may be secondary to high placental resistance caused by an abnormal placental vascular anatomy and/or an abnormally high vasomotor tone. Elevated resistance may be caused by hypovascularity of the IUGR placenta, which has been observed in some studies (2–4) but not all (5, 6). Or, it may be caused by elevated vascular tone secondary to reduced placental nitric oxide synthase levels (7) and/or elevated fetal plasma endothelin (8, 9) and angiotensin II (10) concentrations, which also has been observed in such pregnancies. In any event, maternal-fetal transfer of substances whose transfer is flow-limited (e.g. oxygen) would be reduced by low umbilical arterial blood flows. Indeed, oxygen and glucose extraction from the maternal circulation by the placenta in IUGR pregnancies is impaired to the extent that maternal uterine vein oxygen tensions and glucose concentrations may be abnormally high (11, 12), even though the fetuses themselves may be hypoxic and/or hypoglycemic (13, 14). Fetal hypoxia, in turn, could explain the higher than normal umbilical arterial and venous lactate concentrations sometimes observed in IUGR fetuses (13–15).
On the other hand, low umbilical blood flow cannot explain abnormalities in transfer of other substrates, such as amino acids, that are actively transported from the maternal to fetal circulation by the placenta. Amino acid concentrations are often reduced in plasma of IUGR fetuses, despite maternal concentrations that are as high or higher than those of controls (16, 17). This may be due to reduced amino acid transporter activity, in that transport of the nonmetabolizable neutral amino acid analogs, AIB and methylaminoisobutyric acid (18), is reduced in placental microvillous membrane vesicles isolated from IUGR placentas (19–21).
The objective of the current study was to measure fetoplacental vascular resistance and vascular reactivity, and maternofetal transfer of oxygen, glucose, AIB, and antipyrine in perfused cotyledons from IUGR fetuses with absent or reversed umbilical arterial EDV, compared with preterm and term normally grown controls. We hypothesized that fetoplacental resistance would be elevated and transport reduced in placentas from IUGR pregnancies.
METHODS
Placentas were collected at delivery from term, normally grown fetuses (TN;n = 10), preterm, normally grown fetuses (PN) with normal umbilical arterial EDV (30.8 ± 0.8 wk, n = 6), and preterm IUGR fetuses (<3rd centile) (PI) with absent or reversed umbilical arterial EDV (30.8 ± 0.6 wk, n = 6). All fetuses were morphologically normal. Complete placentas were weighed after the membranes and cord were removed. Clinical data for the three groups are shown in Table 1. Consent was obtained from the mother for the use of placental tissue and access to medical records. The protocol was approved by the University of Toronto Human Ethics Committee. Within 20 min, a peripheral cotyledon was cannulated and the maternal and fetal circulations perfused, by methods described previously (22).
The perfusion medium consisted of Earle's balanced salt solution, with added L-arginine (0.07 g/L; Sigma Chemical Co., St. Louis, MO) and dextran (30 g/L fetal and 7.5 g/L maternal), MW 35,000–50,000 (United States Biochemical, Cleveland, OH). Glucose (0.6 g/L) and heparin (2 USP units/mL; Hepalean, Organon Teknika, Toronto, ON, Canada) were added to the fetal perfusate. Glucose (1 g/L), antipyrine (30 mg/L), and AIB (28 mg/L; Sigma Chemical Co.) were added to the maternal perfusate. Perfusate pH was adjusted to 7.34–7.38 (fetal) and 7.36–7.42 (maternal) with bicarbonate buffer. The maternal perfusate was equilibrated with 95% O2/5% CO2, and the fetal perfusate was equilibrated with 94% N2/6% CO2 (Praxair Products, Mississauga, ON, Canada), except during hypoxia (see below). A miniature temperature probe (Physiotemp, Clifton, NJ) was inserted into the intervillous space, and temperature was maintained between 36°C and 37°C.
The flow rate of the fetal perfusion pump (Masterflex, Cole Parmer Chicago, IL) was adjusted at the start of the stabilization period to achieve a fetal perfusion pressure of 20–30 mm Hg (equivalent to cotyledon perfusion pressure in sheep (23). Flows were then maintained constant for the remainder of the stabilization period and the experiment. Fetal perfusion pressure was measured with an inline pressure transducer (CDXIII; Cobe Laboratories, Lakewood, CO) and was recorded on a chart recorder (Soltec Primeline, Sun Valley, CA). The cotyledon was allowed to stabilize for 45–60 min before beginning the baseline period. Experiments were abandoned if fetal artery pressure was not stable to within 2 mm Hg during this interval, or if, at any point during the study, leakage from the fetal to the maternal side was detected (rate of fetal venous return fell and/or fluid accumulated on the surface of the cotyledon). Leakage in the other direction (i.e. a rise in fetal venous return) was never observed. Mean perfusate flow rates were 0.25 mL min−1 g−1 (fetal) and 0.5 mL min−1 g−1 (maternal), measured with ball-in-tube flowmeters (Gilmont Instruments, Barrington, IL) that were calibrated by timed collection and expressed relative to cotyledon weight determined at the end of the study.
Experiments began with a 30-min baseline period. Perfusion pressure was recorded, and maternal and fetal arterial and venous perfusate samples were collected near the beginning and end of this interval (time-points B1 and B2). Maternal perfusion was then switched to perfusate equilibrated with a gas mixture of 5% CO2/95% N2 to induce a 20-min hypoxic period. Pressures and perfusate samples were collected near the middle and near the end of hypoxia (time-points H1 and H2). Maternal perfusion with the oxygenated perfusate was then resumed, and the cotyledon was allowed to recover for 40 min. This was followed by a second 30-min baseline period with pressure recorded and perfusate samples collected near the beginning and end of this interval (time-points B3 and B4). In most experiments, nitroglycerin (GTN; Sabex, Boucherville, QC, Canada) was then infused for 20 min into the fetal arterial line at an infusion rate of <1% of the fetal arterial flow rate. The final fetal arterial concentration achieved was 10−5 M, a concentration sufficient to induce maximal relaxation (24). Then, after a 20-min recovery period, angiotensin II (0.05 μg; Sigma Chemical Co.) was injected as a bolus into the fetal artery to induce vasoconstriction.
Fetoplacental vascular resistance was calculated as the fetal perfusion pressure divided by the fetal flow rate. Gas tensions and pH were measured with a pH/blood gas analyzer (model 170; Corning Medical, Medfield, MA) within 60 s of sampling. Samples of perfusate were stored frozen (−20°C) until analysis for glucose (YSI model 27; Yellow Springs Instruments, Yellow Springs, OH), lactate, antipyrine, and AIB concentrations. Lactate concentration was measured colorimetrically using a kit based on the conversion of NAD to NADH (Sigma Chemical Co.) on a narrow band spectrophotometer (Shimadzu type UV1600; Mandel Scientific, Toronto, ON, Canada). Antipyrine was also assayed by a colorimetric method (25). AIB concentration was measured with an automated HPLC (model 1050, Hewlett-Packard, ON, Canada) equipped with a fluorescence detector and linear chart recorder. Precolumn derivitization with o-phthaldehyde was used with a mobile phase of 25% acetonitrile in 75% acetate buffer pH 7.2 at a flow rate of 0.6 mL/min. The column was of C18 reversed phase (200 × 4.6 mm with a nominal particle size of 5 μm). Detection was at excitation frequency 365 nm and emission frequency 410 nm.
At the end of the experiment, the cotyledon that had been perfused during the study was fixed by perfusion with 2.5% glutaraldehyde, then dissected out, dry blotted, and the wet weight determined. The entire cotyledon was cut into ∼5-mm cubes that were immersed in fixative in coded vials. Without breaking the code, we randomly selected three cubes from each vial, which were sent to a blinded observer (C.D. Pfarrer) who used scanning electron microscopy to evaluate the villous anatomy according to previously established criteria (2).
Net transfer and consumption by the cotyledon was determined as previously described in detail (22) from concentrations (C) and flows (Q) measured in maternal (M) and fetal (F) arterial (A) and venous (V) catheters where transfer = [(CFV –CFA) × QF] and consumption = [CMA–CMV) × QM −transfer]. Clearances (= transfer/CMA) of AIB and antipyrine were calculated as previously described (26). Transport and consumption values were expressed per unit cotyledon wet weight. ANOVA (Statview 4.5, Abacus Concepts, Berkeley CA), with repeated measures when appropriate, was used to test for differences between the three groups. When group or group-time interactions were significant, separate ANOVAs were performed to compare PI and PN groups and PN and TN groups. Fisher's exact test was used to test proportion for significant differences between groups. Comparisons between PI and TN groups were not made. Baseline measurements refer to B1, B2, B3, and B4 values. Results are presented as mean ± SE where n is the number of placentas.
RESULTS
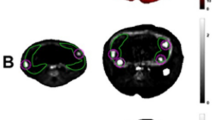
The preterm IUGR group did not differ significantly in gestational age at delivery from the preterm normally grown group, whereas birth weights and placental weights were significantly reduced (Table 1). Of interest was the fact that the weight of the perfused cotyledon did not differ significantly between groups (PI = 14 ± 3 g; PN = 19 ± 3 g, TN = 20 ± 2 g), nor did fetal perfusion rate (PI = 4.1 ± 0.4 mL min−1; PN = 4.1 ± 0.6 mL min−1, TN = 4.9 ± 0.3 mL min−1) or fetal arterial perfusion pressure (PI = 22.7 ± 0.4 mm Hg; PN = 23.9 ± 0.8 mm Hg, TN = 24.0 ± 0.6 mm Hg at B1). Groups also did not differ significantly from the preterm normally grown group values for fetal arterial perfusate PO2 (27.2 ± 0.8 mm Hg at B1), or for maternal perfusion rate (13.8 ± 0.4 mL/min) and maternal arterial perfusate PO2, either at baseline (563 ± 23 mm Hg at B1) or during hypoxia (27 ± 2 mm Hg at H1). Random tissue samples obtained from preterm placental cotyledons were examined by a blinded observer and scored according to characteristics indicated in Table 2. Five of six cotyledons in the PN group were assigned a normal morphology, and five of six in the preterm IUGR group were assigned an abnormal morphology by this observer (Table 2). The most striking difference between normal (Fig. 1) and abnormal (Fig. 2) villous anatomy was the marked reduction in terminal villi and terminal villous buds in the preterm IUGR group. Villous buds normally contain the terminal capillary loops (2).
Scanning electron micrographs of terminal placental villi from preterm normally grown fetuses:(a) intermediate villi (IV) branch into multiple villous buds (arrows mark artifacts) (×1000, ID# PN 30 (30 wk);(b) buds (marked by asterisks) at higher magnification (×2500, ID# PN 30). Note the absence of fibrin or wrinkling of syncytiotrophoblast surface. (c) High magnification showing one intermediate villous (IV) and three terminal villous buds covered with microvilli (×5000, ID# PN 18, 34 wk).
Scanning electron micrographs of terminal placental villi from preterm IUGR fetuses. (a) Intermediate villi (IV) branch into terminal villi (TV) that are typically devoid of villous buds, leaving more room for intervillous space (×1000, ID# PI 25, 31 wk). Villous surface wrinkling (arrows) and “microbuds”(arrowhead) were also common. (b) Also observed were abnormally thin terminal villi (arrow) and few normal-sized terminal villi (TV) (×2500, ID# PI 36, 29 wk). (c) Thin terminal villi sometimes had club-shaped tips (arrows) (×2500, ID# PI 25). (d) An intermediate villous showing “microbuds”(asterisks) but normal microvilli on the syncytiotrophoblast surface (×5000, ID# PI 36).
Surprisingly, baseline vascular resistance tended to be lower in the preterm IUGR group (93 ± 6 mm Hg ml−1 min g), although there were no significant differences between the groups (PN = 136 ± 13, TN = 118 ± 7 mm Hg ml−1 min g) (Fig. 3). The three groups did not differ significantly in the change in vascular resistance caused by nitroglycerin or angiotensin II (Fig. 3). The increase in resistance caused by hypoxia was similar in the two preterm groups, but the increase was significantly greater in term compared with preterm normally grown fetuses (p = 0.001) (Fig. 3).
Fetoplacental vascular resistance (mean ± SE). x axis labels indicate measurements obtained during first baseline period (B1, B2), at 10 and 20 min of hypoxia (H1, H2, respectively), during second baseline period (B3, B4), at end of 20-min infusion of 10−5 M glyceryl trinitrate (GTN), 20 min after GTN (B5), and peak resistance after a 0.05-μg angiotensin II bolus (AII). PI = preterm IUGR. PN = preterm normally grown. TN = term normally grown. Break in x axis is due to different N between B1 and B4, and B4 and AII.
There was no significant difference in baseline glucose consumption between preterm (0.21 ± 0.02 μmol min–1 g−1) and term (TN = 0.23 ± 0.02 μmol min–1 g−1) placentas from normally grown fetuses. However, baseline glucose consumption was 2-fold higher in placentas from preterm IUGR (0.44 ± 0.05 μmol min–1 g−1) than in PN fetuses (p = 0.043) (Fig. 4). Baseline oxygen consumption and lactate production tended to be higher in preterm IUGR placentas (Fig. 4), but differences between groups did not reach significance. During hypoxia, there were no significant differences between groups in the significant decrease in oxygen consumption, and significant increases in glucose consumption and lactate production that were observed at this time (Fig. 4). There were also no significant differences between groups in the significant increase in lactate production and oxygen consumption caused by nitroglycerin infusion, although the increase in lactate production was almost significantly greater in placentas from preterm IUGR than in those from preterm normally grown fetuses (p = 0.054) (Fig. 4). Nitroglycerin did not significantly alter placental glucose consumption.
Placental oxygen consumption, glucose consumption, and lactate production (mean ± SE). x axis labels and group labels are as in Figure 3, except G = GTN. Break in x axis is due to different N between B1 and B4, and B4 and G. N were as shown on Figure 3, except for glucose consumption by TN placentas where N = 8 from B1 to B4, and N = 6 from B4 to G.
Glucose transfer, antipyrine clearance, and lactate production decreased significantly after the first baseline measurement (B1), then remained essentially constant for the remaining baseline measurements, whereas oxygen transfer significantly decreased after hypoxia (B1 and B2 > B3 and B4;p < 0.0001;Fig. 5) with no significant differences between groups. Baseline measurements of glucose transfer, oxygen transfer, and antipyrine clearance did not differ significantly between groups (Fig. 5). During hypoxia, there were no significant differences between groups with regard to the significant decrease in oxygen transfer, glucose transfer, and antipyrine clearance observed at this time (Fig. 5). Nitroglycerin infusion significantly decreased oxygen transfer by 30%—a result that is similar to previous findings in term placentas (22). This response tended to be greatest in preterm control placentas, although the response did not differ significantly between groups (Fig. 5). Nitroglycerin also significantly decreased antipyrine clearance by ∼35% and, again, the response tended to be greatest in the preterm control group, but differences between groups were not significant (Fig. 5). A small decrease in glucose transfer (∼14%) during nitroglycerin was not significant.
Maternofetal oxygen transfer, glucose transfer, and antipyrine clearance (mean ± SE). The x axis labels and group labels are as in Figure 3, except G = GTN. Break in x axis is due to different N between B1 and B4, and B4 and G. N were as shown on Figure 3, except for glucose transfer by TN placentas, where N = 8 from B1 to B4 and N = 6 from B4 to G.
Clearance of AIB was measured at four time points in the protocol (B1, H2, B3, and nitroglycerin). Clearance did not change significantly from baseline values [PI = 0.08 ± 0.01 (n = 6), PN = 0.07 ± 0.01 (n = 6), TN = 0.07 ± 0.01 (n = 8) mL min –1 g−1] during hypoxia or nitroglycerin infusion, nor did clearances differ significantly between groups.
DISCUSSION
Contrary to our hypothesis, baseline fetoplacental vascular resistance in the perfused placental cotyledons of severely IUGR pregnancies with absent or reversed umbilical arterial EDV was not higher than those obtained from normally grown preterm controls. These data are in agreement with the normal perfusion rates and pressures previously reported for perfused cotyledons from IUGR pregnancies with absent umbilical arterial EDV (27). We recognize that cotyledons were not randomly selected for perfusion. However, evaluation by a blinded observer showed that five of six cotyledons that had been perfused in the study in the PI group showed the same abnormalities in villous structure previously described for random samples obtained from entire IUGR placentas (2). The observed reduction in the number of terminal villi and villous buds suggests that the cotyledons chosen for perfusion probably also had the reduced vascularity often observed in IUGR placentas (2–4).
In the current study, the anatomic abnormalities observed in the cotyledons of severely IUGR pregnancies with absent or reversed umbilical arterial EDV were not associated with an elevation in fetoplacental vascular resistance when expressed per g cotyledon wet weight and measured in vitro. We believe that inaccuracies associated with the measurement of fetoplacental vascular resistance in vitro and/or possible intergroup differences in wet-to-dry-weight ratios cannot entirely explain this result. At the perfusion pressure (24 mm Hg) used in our study, the fetoplacental flow rate per g cotyledon weight (0.25 mL min−1 g−1) would be equivalent to a flow rate of 45 mL min−1 kg−1 birth weight in the term normally grown group. This flow rate is ∼60% less than that of human fetuses at term measured in vivo by Doppler ultrasound (110 mL min−1 kg−1) (28). Whether lower flows in vitro were due to elevated vascular resistance in vitro and/or to our use of an underestimated perfusion pressure is not known. Nevertheless, very large increases in resistance in the order of 300–500% are required to reduce umbilical arterial EDV to zero, based on fetal sheep experiments (29) and theoretical studies (30). Thus, it is highly unlikely that errors associated with the in vitro perfusion technique could entirely account for resistance in the preterm IUGR group being, on average, the lowest of the three groups.
The lack of elevated cotyledon resistance in the preterm IUGR group may result from the fact that hypovascularity is due primarily to reduced capillarization of IUGR placentas (2, 6) and—because most resistance resides at the arteriolar rather than capillary level in systemic circulations—as a consequence, reduced capillarization may have been insufficient to elevate resistance. It is noteworthy, however, that not all studies find significant vascular abnormalities in IUGR placentae (5, 6), whereas others find abnormalities in IUGR placentas that are independent of the umbilical arterial EDV (31). Indeed, evidence of such independence was observed for one placenta in each of the preterm groups in the current study. In any event, results suggest that abnormal EDV in the umbilical artery in vivo in IUGR is not caused solely by elevated vascular resistance secondary to abnormal placental anatomy.
There is considerable experimental evidence to support the association between absent or reversed EDV in the umbilical artery and elevated vascular resistance in the placental microcirculation (29, 32) or venous outflow tract of the placenta (i.e. the umbilical vein, ductus venosus, and/or hepatic circulation) (33). Thus, there appear to be two possible explanations for the results of the current study: (1) that absent or reversed EDV in the umbilical artery is caused by elevated resistance in the venous outflow tract or (2) that increased resistance in vivo is caused by a vascular stimulus that is absent in our in vitro system. The first possibility, that elevated resistance resides within the venous outflow tract, deserves further study. The second possibility is also interesting. It suggests that the cause of the elevated resistance is not anatomic, and therefore, if the cause were known, the elevated resistance might be reversed by the appropriate therapeutic intervention. Possible causes for such an elevation in resistance include placental hypoxia (22, 34) elevated maternal intervillous pressure (35), or elevated circulating endothelin (8) or angiotensin II (10) concentrations.
This study failed to demonstrate the expected impairment in maternal to fetal transfer of oxygen, glucose, and AIB in preterm IUGR cotyledons. Because maternal and fetal flow rates were the same in all three groups in vitro, the similarity in antipyrine clearance under baseline conditions suggests that cotyledons in all three groups had similar functional diffusing capacity per g cotyledon weight. This result was surprising, given the obvious anatomic differences we observed in villous anatomy in the IUGR cotyledons (e.g.Fig. 2). Nevertheless, this result suggests that the activities of the neutral amino acid transporters for AIB (18) and the glucose transporter (likely primarily GLUT1 (36) are indistinguishable from normal in IUGR placental tissue when expressed on a per weight basis and studied under in vitro conditions. Normal AIB transport in the current study contrasts with reduced amino acid concentrations in plasma of IUGR fetuses in vivo (16, 17) and with reduced AIB and methylaminoisobutyric acid transport in microvillous membrane vesicles isolated from IUGR placentas studied in vitro (19–21). The reason for the discrepancy in results is not known. In vivo, net transport of glucose and amino acids may be lower in IUGR because of the smaller size of the placenta. Furthermore, the activity of the transporters might be influenced by circulating substances (e.g. hormones or competitive inhibitors) which were not present in the simple perfusion medium used in our in vitro study.
Despite constant flows, antipyrine transfer and oxygen transfer decreased significantly during both hypoxic vasoconstriction and nitroglycerin-induced vasodilation in all three groups. Transfer was presumably reduced during hypoxia by the reduction in vessel surface area caused by vasoconstriction. Nitroglycerin, however, presumably increased surface area by dilating vessels. However, the indiscriminate vasodilation caused by nitroglycerin may have disrupted normal maternal-fetal flow matching, thereby reducing transfer as suggested previously (22). This observation may have clinical significance, because nitroglycerin has been proposed as a therapy for IUGR (37). Although this treatment may well “improve” umbilical arterial Doppler waveforms by transiently decreasing placental resistance, the vasodilation of fetal vessels that were constricted because they were poorly perfused on the maternal side may actually reduce placental transfer and, therefore, be harmful rather than helpful to the fetus.
Glucose consumption by preterm IUGR placentas was twice that of preterm controls in the current study. The placenta is a major consumer of the glucose taken up from the maternal circulation (26), so that an elevated placental consumption of glucose could contribute to the greater difference in glucose concentration between the maternal and fetal circulation in IUGR fetuses with abnormal umbilical arterial EDV (12). However, in the current study, glucose transfer to the fetal side was not diminished. Instead, increased placental glucose consumption was sustained by increased uptake of glucose from the maternal side. Possibly, glucose transport capacity is so high that doubling placental glucose consumption has an insignificant effect on placental glucose transfer. This suggestion is consistent with normal glucose transporter protein levels in IUGR placentas (36, 38).
In the current study, glucose and oxygen consumption per g remained constant with development from preterm to term in normally grown placentas. This result is in accord with results from large fragments of placental tissue incubated in vitro, but it contrasts with results obtained from small fragments, in which placental glucose and oxygen consumption decreased with advancing gestation (39). As shown previously in the perfused human cotyledon at term, hypoxia increased fetoplacental vascular resistance (34), glucose consumption, and lactate production, and it decreased oxygen consumption (40). The current study further shows that the vasoconstrictive response to hypoxia increased, whereas the metabolic effects remained unchanged with increasing gestation.
In summary, fetoplacental resistance per g cotyledon weight was not elevated in cotyledons from preterm IUGR fetuses with absent or reversed EDV in the umbilical artery relative to preterm controls, even though anticipated differences in villous anatomy were observed. These data suggest that reduced umbilical arterial EDV in such fetuses is not due to an elevation in placental microvascular resistance secondary to an anatomic abnormality. Instead, reduced umbilical arterial EDV may be caused by an elevated resistance in the umbilical venous outflow tract, or by elevated placental resistance caused by a vascular stimulus that is absent in vitro. Net maternofetal transfer of oxygen and glucose, and clearance of aminoisobutyric acid and antipyrine per g cotyledon weight, were also normal in IUGR placentas. However, IUGR placentas were not entirely normal in vitro. Glucose consumption was doubled in preterm IUGR placentas relative to preterm controls. These data suggest that reduced substrate delivery in IUGR fetuses is due to the reduced weight of the IUGR placenta, abnormal fetal and/or maternal flow rates, abnormal fetal and/or placental metabolism, or to the influence of substances not present in our simple perfusion medium.
Abbreviations
- AIB:
-
aminoisobutyric acid
- EDV:
-
end-diastolic velocity
- IUGR:
-
intrauterine growth restriction
- PI:
-
preterm IUGR
- PN:
-
preterm normally grown
- TN:
-
term normally grown
References
Gill RW, Kossoff G, Warren PS, Garrett WJ 1984 Umbilical venous flow in normal and complicated pregnancy. Ultrasound Med Biol 10: 349–363
Krebs C, Macara LM, Leiser R, Bowman AW, Greer IA, Kingdom JC 1996 Intrauterine growth restriction with absent end-diastolic flow velocity in the umbilical artery is associated with maldevelopment of the placental terminal villous tree. Am J Obstet Gynecol 175: 1534–1542
Giles WB, Trudinger BJ, Baird PJ 1985 Fetal umbilical artery flow velocity waveforms and placental resistance: pathological correlation. Br J Obstet Gynaecol 92: 31–38
McCowan LM, Mullen BM, Ritchie K 1987 Umbilical artery flow velocity waveforms and the placental vascular bed. Am J Obstet Gynecol 157: 900–902
Jackson MR, Walsh AJ, Morrow RJ, Mullen JBM, Lye SJ, Ritchie JWK 1995 Reduced placental villous tree elaboration in small-for-gestational-age pregnancies: relationship with umbilical artery Doppler waveforms. Am J Obstet Gynecol 172: 518–525
Macara L, Kingdom JCP, Kohnen G, Bowman AW, Greer IA, Kaufmann P 1995 Elaboration of stem villous vessels in growth restricted pregnancies with abnormal umbilical artery Doppler waveforms. Br J Obstet Gynaecol 102: 807–812
Morris NH, Sooranna SR, Learmont JG, Poston L, Ramsey B, Pearson JD, Steer PJ 1995 Nitric oxide synthase activities in placental tissue from normotensive, pre-eclamptic and growth retarded pregnancies. Br J Obstet Gynaecol 102: 711–714
Harvey-Wilkes KB, Nielsen HC, D'Alton ME 1996 Elevated endothelin levels are associated with increased placental resistance. Am J Obstet Gynecol 174: 1599–1604
McQueen J, Kingdom JCP, Connell JMC, Whittle MJ 1993 Fetal endothelin levels and placental vascular endothelin receptors in intrauterine growth retardation. Obstet Gynecol 82: 992–998
Kingdom JCP, McQueen J, Connell JMC, Whittle MJ 1993 Fetal angiotensin II levels and vascular (type I) angiotensin receptors in pregnancies complicated by intrauterine growth retardation. Br J Obstet Gynaecol 100: 476–482
Pardi G, Cetin I, Marconi AM, Bozzetti P, Buscaglia M, Makowski EL, Battaglia FC 1992 Venous drainage of the human uterus: respiratory gas studies in normal and fetal growth-retarded pregnancies. Am J Obstet Gynecol 166: 699–706
Marconi AM, Paolini C, Buscaglia M, Zerbe G, Battaglia FC, Pardi G 1996 The impact of gestational age and fetal growth on the maternal-fetal glucose concentration difference. Obstet Gynecol 87: 937–942
Nicolaides KH, Economides DL, Soothill PW 1989 Blood gases, pH, and lactate in appropriate- and small-for-gestational-age fetuses. Am J Obstet Gynecol 161: 996–1001
Economides DL, Nicolaides KH, Campbell S 1991 Metabolic and endocrine findings in appropriate and small for gestational age fetuses. J Perinat Med 19: 97–105
Marconi AM, Cetin I, Ferrazzi E, Ferrari MM, Pardi G, Battaglia FC 1990 Lactate metabolism in normal and growth-retarded human fetuses. Pediatr Res 28: 652–656
Economides DL, Nicolaides KH, Gahl WA, Bernardini I, Evans MI 1989 Plasma amino acids in appropriate- and small-for-gestational-age fetuses. Am J Obstet Gynecol 161: 1219–1227
Cetin I, Ronzoni S, Marconi AM, Perugino G, Corbetta C, Battaglia FC, Pardi G 1996 Maternal concentrations and fetal-maternal concentration differences of plasma amino acids in normal and intrauterine growth-restricted pregnancies. Am J Obstet Gynecol 174: 1575–1583
Józwik M, Teng C, Timmerman M, Chung M, Meschia G, Battaglia FC 1998 Uptake and transport by the ovine placenta of neutral nonmetabolizable amino acids with different transport system affinities. Placenta 19: 531–538
Dicke JM, Henderson GI 1988 Rapid communication: placental amino acid uptake in normal and complicated pregnancies. Am J Med Sci 295: 223–227
Glazier JD, Cetin I, Perugino G, Ronzoni S, Grey AM, Mahendran D, Marconi AM, Pardi G, Sibley CP 1997 Association between the activity of the system A amino acid transporter in the microvillous plasma membrane of the human placenta and severity of fetal compromise in intrauterine growth restriction. Pediatr Res 42: 514–519
Mahendran D, Donnai P, Glazier JD, D'Souza SW, Boyd RDH, Sibley CP 1993 Amino acid (system A) transporter activity in microvillous membrane vesicles from the placentas of appropriate and small for gestational age babies. Pediatr Res 34: 661–665
Byrne BM, Howard RB, Morrow RJ, Whiteley KJ, Adamson SL 1997 Role of the L-arginine nitric oxide pathway in hypoxic fetoplacental vasoconstriction. Placenta 18: 627–634
Adamson SL, Whiteley KJ, Langille BL 1992 Pulsatile pressure-flow relations and pulse-wave propagation in the umbilical circulation of fetal sheep. Circ Res 70: 761–772
Myatt L, Brewer A, Brockman DE 1991 The action of nitric oxide in the perfused human fetal-placental circulation. Am J Obstet Gynecol 164: 687–692
Brodie BB, Axelrod J, Soberman R, Levy BB 1949 The estimation of antipyrine in biological materials. J Biol Chem 179: 25–29
Schneider H, Challier J-C, Dancis J 1981 Transfer and metabolism of glucose and lactate in the human placenta studied by a perfusion system in vitro. In: Young M, Boyd RDH, Longo LD, Telegdy G (eds) Placental Transfer: Methods and Interpretation. Placenta (Supplement 2). W.B. Saunders Company Ltd., London, East Sussex, pp 129–137
Clifton VL, Read MA, Leitch IM, Giles WB, Boura ALA, Robinson PJ, Smith R 1995 Corticotropin-releasing hormone-induced vasodilation in the human fetal-placental circulation involvement of the nitric oxide-cyclic guanosine 3′,5′-monophosphate-mediated pathway. J Clin Endocrinol Metab 80: 2888–2893
St John Sutton MS, Theard MA, Bhatia SJS, Plappert T, Saltzman DH, Doubilet P 1990 Changes in placental blood flow in the normal human fetus with gestational age. Pediatr Res 28: 383–387
Adamson SL, Morrow RJ, Langille BL, Bull SB, Ritchie JWK 1990 Site-dependent effects of increases in placental vascular resistance on the umbilical arterial velocity waveform in fetal sheep. Ultrasound Med Biol 16: 19–27
Surat DR, Adamson SL 1996 Downstream determinants of pulsatility of the mean velocity waveform in the umbilical artery as predicted by a computer model. Ultrasound Med Biol 22: 707–717
Salafia CM, Pezzullo JC, Minior VK, Divon MY 1997 Placental pathology of absent and reversed end-diastolic flow in growth-restricted fetuses. Obstet Gynecol 90: 830–836
Morrow RJ, Adamson SL, Bull SB, Ritchie JWK 1989 Effect of placental embolization on the umbilical arterial velocity waveform in fetal sheep. Am J Obstet Gynecol 161: 1055–1060
van Vugt JMG, Hasaart THM, Ruissen CJ, Hoogland HJ, Hoeks APG, de Haan J 1988 Pulsatility index and its relationship to placental vascular resistance during partial umbilical venous occlusion: a study in fetal lambs. Gynecol Obstet Invest 26: 1–7
Howard RB, Hosokawa T, Maguire MH 1987 Hypoxia-induced fetoplacental vasoconstriction in perfused human placental cotyledons. Am J Obstet Gynecol 157: 1261–1266
Karimu AL, Burton GJ 1994 The effects of maternal vascular pressure on the dimensions of the placental capillaries. Br J Obstet Gynaecol 101: 57–63
Jansson T, Wennergren M, Illsley NP 1993 Glucose transporter protein expression in human placenta throughout gestation and in intrauterine growth retardation. J Clin Endocrinol Metab 77: 1554–1562
Thaler I, Amit A, Jakobi P, Itskovitz-Eldor J 1996 The effect of isosorbide dinitrate on uterine artery and umbilical artery flow velocity waveforms at mid-pregnancy. Obstet Gynecol 88: 838–843
Kainulainen H, Jarvinen T, Heinonen PK 1997 Placental glucose transporters in fetal intrauterine growth retardation and macrosomia. Gynecol Obstet Invest 44: 89–92
Malek A, Sager R, Altermatt HJ, Gaeng D, Leiser R, Schneider H 1996 Glucose consumption and lactate production of human placental tissue under different conditions of in vitro incubation. J Soc Gynecol Investig 3: 113–120
Illsley NP, Penfold P, Bardsley SE, Tracey BM, Aarnoudse JG 1983 The effects of anoxia on human placental metabolism and fetal substrate profiles investigated by an in vitro placental perfusion technique. Trophoblast Res 1: 55–70
Acknowledgements
The authors thank Kathey Whiteley, Brenda Knie, Alexsandra Pastrakuljic, Carmine Simone, and Bridgette Byrne for their advice and technical support, and Dr. Angelo Tesoro for HPLC analysis of AIB concentrations. We also thank Lindsay McWhirter and the delivery suite staff of Mount Sinai Hospital for their invaluable assistance in obtaining placentas.
Author information
Authors and Affiliations
Additional information
Supported by a grant from the Medical Research Council of Canada. S.L.A. received support as a Career Investigator of the Heart and Stroke Foundation of Ontario.
Rights and permissions
About this article
Cite this article
Challis, D., Pfarrer, C., Ritchie, J. et al. Glucose Metabolism Is Elevated and Vascular Resistance and Maternofetal Transfer Is Normal in Perfused Placental Cotyledons from Severely Growth-Restricted Fetuses. Pediatr Res 47, 309–315 (2000). https://doi.org/10.1203/00006450-200003000-00005
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-200003000-00005